

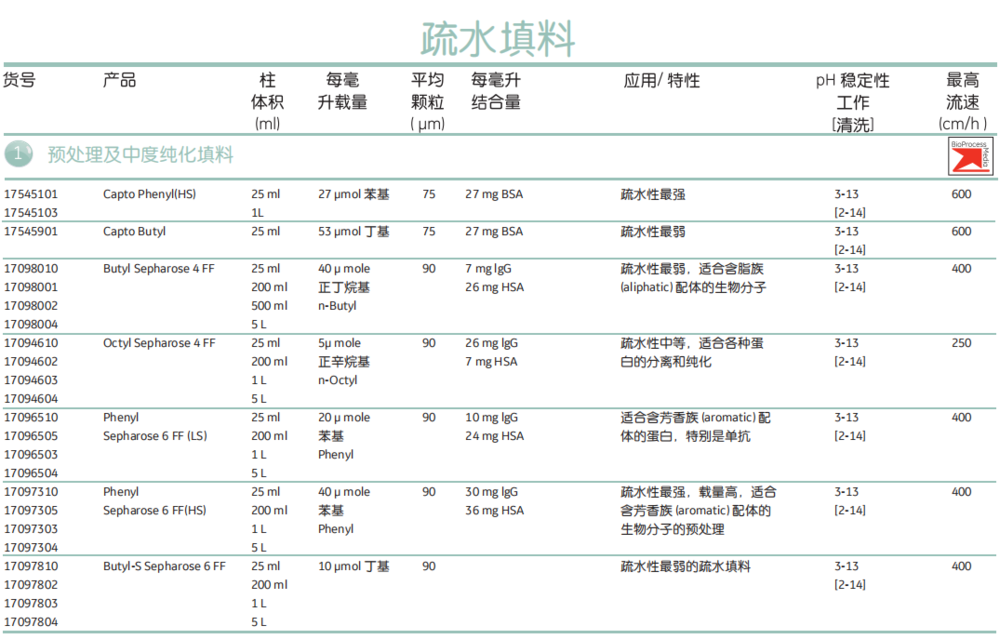
HP-SEC analysis showed a geometrically symmetrical peak, indicating rhFTH exists as homogeneous structure with a relatively large size, and further being validated by DLS and TEM. Circular dichroism analysis revealed alpha-helix structure as major structure content and fluorescence analysis demonstrated that aromatic amino acids residues were highly buried inside. ESI-MS analysis showed a molecular weight of 21086.0 Da that was almost identical with the theoretical value. Combining with hydrophobic interaction chromatography of Capto Butyl, the rhFTH was efficiently purified from the post-precipitated supernatant, the purity of the finally obtained rhFTH was above 98 % and the target protein recovery was around 66 % roughly estimated by optical density. The resins are used in many applications, including the purification of: Plasmids and viral vectors for gene therapy Recombinant proteins Monoclonal antibodies (mAb), Fab antibodies, and bispecific antibodies (bsAb). Almost all of the unrelated proteins could be efficiently removed by the optimized precipitation procedure. The high chemical stability of Capto resins lets you perform cleaning-in-place procedures with ease. Three factors including heating temperature, pH value and sodium chloride concentration were systematically investigated and optimized for this precipitation procedure. Capto Phenyl (high sub) and Capto Butyl are hydrophobic interaction chromatography (HIC) resins used in the capture and intermediate stages of protein purification. To establish a robust purification scheme, we developed a non-chromatographic precipitation procedure as a key step for extracting rhFTH from the disrupted supernatant.
1-9 ISSN: 1359-5113 Subject: Escherichia coli, absorbance, circular dichroism spectroscopy, ferritin, fluorescence, humans, hydrophobic interaction chromatography, molecular weight, pH, purification methods, sodium chloride, temperature Abstract: Recombinant human ferritin heavy chain (rhFTH) was highly expressed and self-assembled in E. Successful implementation of the proposed scheme has been demonstrated for two different monoclonal antibody therapeutic products.Development of robust and facile purification process for production of recombinant human ferritin heavy chain nanoparticle from Escherichia coli Author: Xiaotong Song, Yongxiang Zheng, Ling Zhu, Li Zhang, Huan Meng, Rong Yu, Chun Zhang Source: Process biochemistry 2021 v.104 pp. Further, clearance of host cell proteins was also shown to have improved with the suggested process scheme. 1–2%) at >95% recovery and reduced overall process time (6 h vs. The proposed process scheme yielded improved separation of aggregates (0% vs. Hydrophobicity Order of the four HIC NP phase: Ethyl


 0 kommentar(er)
0 kommentar(er)
